Our Expertise In Export
Highly Efficient Manufacturing Facility
Our pharmaceutical products are manufactured in highly efficient, state-of-the-art plants, ensuring premium quality and safety. We proudly export our medicines to numerous countries, meeting international healthcare standards with reliability and excellence.
WHO-GMP Certified Facility


WHO-GMP certified facility ensures all our products are manufactured in highest quality standards and internationally accepted.




ISO Certified Facility
International Organization for Standardization (ISO) certified plant to ensure credibility and regulatory compliance.
Good Laboratory Practices (GLP) certified facility ensures quality, reliability and operational efficiency.
GLP Certified Facility


Dedicated Export Team
Our dedicated export team is committed to ensuring smooth and efficient export operations. With expertise in international regulations, logistics, and compliance, our team manages the entire process—from documentation to product registration and distribution. We work closely with clients and regulatory authorities to ensure timely deliveries while adhering to the highest quality standards. Whether it's navigating complex market requirements or supporting new registrations, our export team is here to facilitate your business growth globally.
Regulatory Knowledge and Market Readiness
With a deep understanding of international regulatory frameworks, we ensure full compliance across diverse markets. Our team is well-versed in handling market-specific documentation, product registrations, and certifications required for smooth entry into global regions. By staying updated with the latest regulations and industry standards, we are fully prepared to meet the demands of different countries and offer flexible solutions for product export and distribution worldwide. This readiness enables us to cater to diverse markets with ease and efficiency.
International Standards Compliance
At Caplet Health Care, we ensure that all our pharmaceutical products are manufactured in compliance with international standards such as WHO-GMP, ISO, and GLP certifications. This commitment to global quality benchmarks guarantees that our products meet the stringent requirements of international markets, ensuring safety, efficacy, and reliability. Our adherence to these standards reflects our dedication to providing high-quality healthcare solutions worldwide.
Regulatory Compliance
At Caplet Health Care, our manufacturing facility is fully compliant with international standards, including WHO-GMP, ISO, and GLP certifications. Our facility is already registered with several countries, including Uzbekistan, Georgia, Myanmar, Philippines, Kenya, Uganda, Niger, Ghana among several others. Additionally, we are in the process of expanding our reach, with several countries under registration where our plant is either under inspection or awaiting certification. This underscores our commitment to delivering quality healthcare solutions across global markets.
Our Registrations
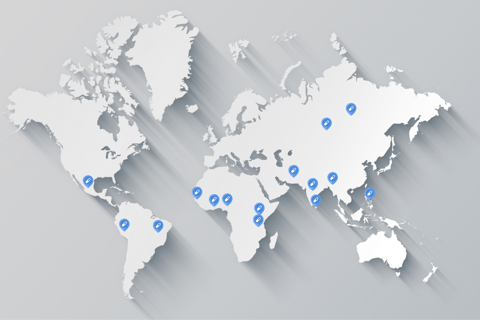
Global Presence
Caplet Health Care operates in key markets worldwide, ensuring quality medicines are accessible to communities in need across various regions.
The state-of-the-art manufacturing plants are registered in key markets, including Kenya, Ghana, South Africa, Nigeria, Iraq, Yemen, Myanmar, the Philippines, Nepal, and Sri Lanka, among others.
Enquire now to become a part of our global partner!